Chapter 6 review chemical bonding answer key Oliver, Middlesex County

Math tutor Math Tutor Drawing Lewis Structures Answers chapter 6 review chemical bonding answer key PDF is available on our online library. With our online resources, you can find chapter 6 review chemical bonding answer key or just about any type of ebooks, for any type of product. Best of all, they are entirely free to find, use and download, so there is no cost or stress at all. chapter 6 review chemical bonding answer key PDF may not make
Worksheet Answer Keys Chemistry - Google
Chapter 6 Chemical Bonds Amazon S3. Chapter 6: Chemical Bonds Review Answer Key. What is the most stable group on the Periodic Table? Why is this group stable? Noble Gases (Group 18) because they have 8 valence electrons which means their outer energy level is full. Name the most reactive group(s) on …, Unit 13: Thermochemistry Chapter 11 Review Packet Answer Key.pdf. (467k) Chapter 11 Section 1_Describing Chemical Reactions.ppt. (1255k). Chapter 11 Chemical Reactions Assessment Answers, Chapter 8 Section 1 Chemical Equations And Reactions, Chemistry Download chapter 11 chemical reactions packet answers ebooks PDF file for free, Get many PDF.
Review Previous Concepts Chemical Bonding CHAPTER 6 Section 1 Introduction to Chemical Bonding What is a chemical bond and why does it form? Section 2 Covalent Bonding and Molecular Compounds What is a molecular formula? What are the characteristics of a covalent bond? Start studying Chemistry Chapter 6: Chemical Bonding Test Study Guide. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Interactive Reader and Study Guide 1 The Nature of Physical Science SECTION1 Science and Scientists The Nature of Physical Science Name Class Date CHAPTER 1 After you read this section, you should be able to answer these questions: • What are three methods used by scientists to conduct investigations? • How does science help people? chapter 6 review chemical bonding section 3 answers.pdf FREE PDF DOWNLOAD NOW!!! Source #2: chapter 6 review chemical bonding section 3 answers.pdf
Chapter 13 Chemical Bonding 13.1 – Electrons & Chemical Bonding Directed Reading Worksheet “Comparing Integers on a Number Line” WS “Arithmetic w/ Positive & Negative Numbers” WS Section Review p.331 Quiz 13.2 – Types of Chemical bonds Directed Reading Worksheet Math Break p.334 Section Review p.335 Start studying Chapter 6 Review: Chemical Bonding. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Accelerated Chemistry 1 Chemical Bonding Chapter 6 PRETEST: Chemical Bonding In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive. b. always negative. CHAPTER 6 TEST: CHEMICAL BONDING REVIEW SHEET. 1. What are the 2 main types of bonds that we have learned about? 2. During an ionic bond, valence electrons are_____ 3. During a covalent bond, valence electrons are_____ 4. What periodic trend can be used to determine the type of bond that elements will form? 1. IONIC AND COVALENT. 2. TAKEN OR GIVEN AWAY. 3. SHARED. 4. …
The Chemical Bonding chapter of this Holt McDougal Modern Chemistry Companion Course helps students learn the essential lessons associated with chemical bonding. Review Previous Concepts Chemical Bonding CHAPTER 6 Section 1 Introduction to Chemical Bonding What is a chemical bond and why does it form? Section 2 Covalent Bonding and Molecular Compounds What is a molecular formula? What are the characteristics of a covalent bond?
Physical Science Reading and Study Workbook Level B Chapter 6 55 IPLS Chapter 6 Chemical Bonds Summary 6.1 Ionic Bonding When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react. • The chemical properties of an element depend on the number of valence electrons. Hydrogen bonding is than Van der Waals. Requires molecules with hydrogen bonded directly to F, O, or N. Example: Water iOAiC Electrostatic attractions occur between compounds. Metallic bonding is both an intra and an intermolecular bond. Intermolecular bonds between metalloids or elements bordering metalloids are network solids. Covalent Bond
CHAPTER 6 REVIEW Chemical Bonding SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence electrons and of different atoms. (a) nuclei (c) isotopes (b) inner electrons (d) Lewis structures 2. b A covalent bond consists of (a) a shared electron. Unit 13: Thermochemistry Chapter 11 Review Packet Answer Key.pdf. (467k) Chapter 11 Section 1_Describing Chemical Reactions.ppt. (1255k). Chapter 11 Chemical Reactions Assessment Answers, Chapter 8 Section 1 Chemical Equations And Reactions, Chemistry Download chapter 11 chemical reactions packet answers ebooks PDF file for free, Get many PDF
Mrs. Packard's Chemistry Classes. Home. Advanced Chemistry pd 1. Chapter 10. Chapter 11. Chapter 12. Chapter 13. Chapter 15. Chapter 16. Chapter 17. Fall Semester . Spring Final Exam Info. AP Chemistry pd 2 and 4. Chapter 11. Chapter 12. Chapter 13. Chapter 14. Chapter 15 and 16.1. Chapter 17. Chapter 18. Fall Semester. Qualitative Analysis Lab Stuff. Reaction Sets Info. Vacation homework Start studying Chemistry Chapter 6: Chemical Bonding Test Study Guide. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
CHAPTER 7 REVIEW Chemical Formulas and Chemical Compounds SECTION 2 SHORT ANSWER Answer the following questions in the space provided. 1. Assign the oxidation number to the specified element in each of the following examples: 4 a. S in H 2SO 3 6 b. S in MgSO 4 2 c. S in K 2S 1 d. Cu in Cu 2S 6 e. Cr in Na 2CrO 4 5 f. N in HNO 3 4 g. C in (HCO 3 CHAPTER 6 REVIEW Chemical Bonding SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence electrons and of different atoms. (a) nuclei (c) isotopes (b) inner electrons (d) Lewis structures 2. b A covalent bond consists of (a) a shared electron.
40 vocabulary words NQHS Modern Chemistry Chapter 6 Chemical Bonding Learn with flashcards, games, and more — for free. 40 vocabulary words NQHS Modern Chemistry Chapter 6 Chemical Bonding Learn with flashcards, games, and more — for free.
CHAPTER 6 REVIEW Chemical Bonding Ms. Minor's Webpage. Hydrogen bonding is than Van der Waals. Requires molecules with hydrogen bonded directly to F, O, or N. Example: Water iOAiC Electrostatic attractions occur between compounds. Metallic bonding is both an intra and an intermolecular bond. Intermolecular bonds between metalloids or elements bordering metalloids are network solids. Covalent Bond, Chapter 6: Chemical Bonds Review Answer Key. What is the most stable group on the Periodic Table? Why is this group stable? Noble Gases (Group 18) because they have 8 valence electrons which means their outer energy level is full. Name the most reactive group(s) on ….
Math tutor Math Tutor Drawing Lewis Structures Answers

Chapter 13 Period Chemical Bonding. Start studying Chapter 6 Review: Chemical Bonding. Learn vocabulary, terms, and more with flashcards, games, and other study tools., Chapter 6 Notes - Chemical Bonding Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction to Chemical Bonding I. Types of Chemical Bonding A. Ionic Bonding 1. Chemical bonding that results from the electrical attraction between large numbers of cations and anions 2. Electrons are transferred in.

CHAPTER 6 Chemical Bonding mchsapchemistry.com

www.isd622.org. A chemical bond that forms when atoms share electrons is always a(n) a. polar bond. c. metallic bond. b. ionic bond. d. covalent bond. ____ 5. When two fluorine atoms share a pair of electrons, the bond that forms is a(n) a. polar covalent bond. c. nonpolar covalent bond. b. ionic bond. d. double bond. ____ 6. The chemical formula for magnesium https://upload.wikimedia.org/wikipedia/commons/3/37/High_School_Chemistry_Workbook.pdf Start studying Chapter 6 Review Chemical Bonding Section 2&3. Learn vocabulary, terms, and more with flashcards, games, and other study tools..

Review Answers 1. A chemical bond is a link between atoms resulting from the mutual attraction of their nuclei and electrons. 2. The three major types of chemical bonding are ionic, covalent, and metallic. In ionic bonding, large numbers of oppositely charged ions join because of mutual electrical attraction. In covalent bonding, atoms join by sharing electron pairs. In metallic bonding, atoms Start studying Chapter 6 Review: Chemical Bonding. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Chemical Names and Formulas Section 6.1 Introduction to Chemical Bonding What does it mean to say that an atom is neutral? The number of protons is equal to the number of electrons.Net charge is zero. Chapter 6 Notes - Chemical Bonding Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction to Chemical Bonding I. Types of Chemical Bonding A. Ionic Bonding 1. Chemical bonding that results from the electrical attraction between large numbers of cations and anions 2. Electrons are transferred in
Interactive Reader and Study Guide 1 The Nature of Physical Science SECTION1 Science and Scientists The Nature of Physical Science Name Class Date CHAPTER 1 After you read this section, you should be able to answer these questions: • What are three methods used by scientists to conduct investigations? • How does science help people? Unit 13: Thermochemistry Chapter 11 Review Packet Answer Key.pdf. (467k) Chapter 11 Section 1_Describing Chemical Reactions.ppt. (1255k). Chapter 11 Chemical Reactions Assessment Answers, Chapter 8 Section 1 Chemical Equations And Reactions, Chemistry Download chapter 11 chemical reactions packet answers ebooks PDF file for free, Get many PDF
Start studying Chapter 6 Review Chemical Bonding Section 1. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Modern Chemistry 49 Chemical Bonding CHAPTER 6 REVIEW Chemical Bonding SECTION 5 SHORT ANSWER Answer the following questions in the space provided. 1. Identify the major assumption of the VSEPR theory, which is used to predict the shape of atoms. _____
A chemical bond that forms when atoms share electrons is always a(n) a. polar bond. c. metallic bond. b. ionic bond. d. covalent bond. ____ 5. When two fluorine atoms share a pair of electrons, the bond that forms is a(n) a. polar covalent bond. c. nonpolar covalent bond. b. ionic bond. d. double bond. ____ 6. The chemical formula for magnesium Holt McDougal Modern Chemistry Chapter 6: Chemical Bonding Chapter Exam Instructions. Choose your answers to the questions and click 'Next' to see the next set of questions.
Play a game of Kahoot! here Chapter 6 chemical bonding mixed review answer key. Kahoot! is a free game-based learning platform that makes it fun to learn – any subject, in any language, on any device, for all ages! Chapter 6 chemical bonding mixed review answer key Modern Chemistry 46 Chapter Test Chapter: Chemical Bonding In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. _____ 1. The charge on an ion is a. always positive. b. always negative. c. either positive or negative. d. zero. _____ 2. According to the octet rule, a calcium atom has a tendency to a. lose one electron. b
chapter 6 review chemical bonding section 3 answers.pdf FREE PDF DOWNLOAD NOW!!! Source #2: chapter 6 review chemical bonding section 3 answers.pdf Name KEY Period Date Chapter 6: Review. Bonding Comparison Chart. IONIC COVALENT METALLIC Types of Atoms Involved (Metal, Nonmetal) Metals and nonmetals Nonmetals Metals and metals Method of Bond Formation (Valence Electrons) Positive ions bonding with negative ions…Transfer of Electrons Sharing valence electrons Valence electrons are shared among atoms…A Sea of Electrons Type of …
Chapter 6 Notes - Chemical Bonding Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction to Chemical Bonding I. Types of Chemical Bonding A. Ionic Bonding 1. Chemical bonding that results from the electrical attraction between large numbers of cations and anions 2. Electrons are transferred in CHAPTER 6 REVIEW Chemical Bonding SECTION 5 SHORT ANSWER Answer the following questions in the space provided. 1. Identify the major assumption of the VSEPR theory, which is used to predict the shape of atoms. Pairs of valence electrons repel one another.
Physical Science Reading and Study Workbook Level B Chapter 6 55 IPLS Chapter 6 Chemical Bonds Summary 6.1 Ionic Bonding When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react. • The chemical properties of an element depend on the number of valence electrons. Play a game of Kahoot! here Chapter 6 chemical bonding mixed review answer key. Kahoot! is a free game-based learning platform that makes it fun to learn – any subject, in any language, on any device, for all ages! Chapter 6 chemical bonding mixed review answer key
Chemical Bonding Quiz #1. 1. The number of electrons in the outer shell ; A) isotope B) valence C) ion D) atomic mass. 2. These elements don't bond with other elements because their outer shell is filled. A) metals B) noble solids C) Inert gases D) none of the answers are correct. 3. Most atoms adopt one of three simple strategies to achieve a filled shell. Which of the following is NOT one of Start studying Chapter 6 Review: Chemical Bonding. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Physical Science Reading and Study Workbook Level B Chapter 6 55 IPLS Chapter 6 Chemical Bonds Summary 6.1 Ionic Bonding When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react. • The chemical properties of an element depend on the number of valence electrons. CHAPTER 6 TEST: CHEMICAL BONDING REVIEW SHEET. 1. What are the 2 main types of bonds that we have learned about? 2. During an ionic bond, valence electrons are_____ 3. During a covalent bond, valence electrons are_____ 4. What periodic trend can be used to determine the type of bond that elements will form? 1. IONIC AND COVALENT. 2. TAKEN OR GIVEN AWAY. 3. SHARED. 4. …
Name_________________________________

Chapter 8 9 and 10 Mrs. Packard's Chemistry. Read Online Now section 1 stability in bonding answer key Ebook PDF at our Library. Get section 1 stability in bonding answer key PDF file for free from our online library PDF File: section 1 stability in bonding answer key. to suit your own needs. Here is the access Download Page of SECTION 1 STABILITY IN BONDING ANSWER KEY PDF, click, Unit 13: Thermochemistry Chapter 11 Review Packet Answer Key.pdf. (467k) Chapter 11 Section 1_Describing Chemical Reactions.ppt. (1255k). Chapter 11 Chemical Reactions Assessment Answers, Chapter 8 Section 1 Chemical Equations And Reactions, Chemistry Download chapter 11 chemical reactions packet answers ebooks PDF file for free, Get many PDF.
Chemical bonding test key and worksheets Teaching Resources
Chapter 6 Wordwise Chemical Bonds Answer Key ePub. Google apps. Main menu, CHAPTER 6 REVIEW Chemical Bonding SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence electrons and of different atoms. (a) nuclei (c) isotopes (b) inner electrons (d) Lewis structures 2. b A covalent bond consists of (a) a shared electron..
40 vocabulary words NQHS Modern Chemistry Chapter 6 Chemical Bonding Learn with flashcards, games, and more — for free. A chemical bond that forms when atoms share electrons is always a(n) a. polar bond. c. metallic bond. b. ionic bond. d. covalent bond. ____ 5. When two fluorine atoms share a pair of electrons, the bond that forms is a(n) a. polar covalent bond. c. nonpolar covalent bond. b. ionic bond. d. double bond. ____ 6. The chemical formula for magnesium
Hydrogen bonding is than Van der Waals. Requires molecules with hydrogen bonded directly to F, O, or N. Example: Water iOAiC Electrostatic attractions occur between compounds. Metallic bonding is both an intra and an intermolecular bond. Intermolecular bonds between metalloids or elements bordering metalloids are network solids. Covalent Bond Physical Science Reading and Study Workbook Level B Chapter 6 55 IPLS Chapter 6 Chemical Bonds Summary 6.1 Ionic Bonding When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react. • The chemical properties of an element depend on the number of valence electrons.
CHAPTER 6 REVIEW Chemical Bonding SECTION 5 SHORT ANSWER Answer the following questions in the space provided. 1. Identify the major assumption of the VSEPR theory, which is used to predict the shape of atoms. Pairs of valence electrons repel one another. Modern Chemistry 7 Chemical Bonding CHAPTER 6 STUDY GUIDE Chemical Bonding SECTION 2 COVALENT BONDING AND MOLECULAR COMPOUNDS SHORT ANSWER Answer the following questions in the space provided. 1. Use the concept of potential energy to describe how a covalent bond forms
Physical Science Reading and Study Workbook Level B Chapter 6 55 IPLS Chapter 6 Chemical Bonds Summary 6.1 Ionic Bonding When the highest occupied energy level of an atom is filled with electrons, the atom is stable and not likely to react. • The chemical properties of an element depend on the number of valence electrons. chapter 6 review chemical bonding answer key PDF is available on our online library. With our online resources, you can find chapter 6 review chemical bonding answer key or just about any type of ebooks, for any type of product. Best of all, they are entirely free to find, use and download, so there is no cost or stress at all. chapter 6 review chemical bonding answer key PDF may not make
Start studying Chapter 6 Review Chemical Bonding Section 1. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Chapter 6 Test The Structure of Matter MULTIPLE CHOICE. Write the letter of the term or phrase that best completes each statement or best answers each question on the answer sheet provided. _____1. A compound differs from a mixture because it a. always remains frozen even at high temperatures. b. is formed from two cations.
chapter 6 review chemical bonding answer key PDF is available on our online library. With our online resources, you can find chapter 6 review chemical bonding answer key or just about any type of ebooks, for any type of product. Best of all, they are entirely free to find, use and download, so there is no cost or stress at all. chapter 6 review chemical bonding answer key PDF may not make Modern Chemistry 7 Chemical Bonding CHAPTER 6 STUDY GUIDE Chemical Bonding SECTION 2 COVALENT BONDING AND MOLECULAR COMPOUNDS SHORT ANSWER Answer the following questions in the space provided. 1. Use the concept of potential energy to describe how a covalent bond forms
Modern Chemistry 46 Chapter Test Chapter: Chemical Bonding In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. _____ 1. The charge on an ion is a. always positive. b. always negative. c. either positive or negative. d. zero. _____ 2. According to the octet rule, a calcium atom has a tendency to a. lose one electron. b The Chemical Bonding chapter of this Holt McDougal Modern Chemistry Companion Course helps students learn the essential lessons associated with chemical bonding.
Read Online Now section 1 stability in bonding answer key Ebook PDF at our Library. Get section 1 stability in bonding answer key PDF file for free from our online library PDF File: section 1 stability in bonding answer key. to suit your own needs. Here is the access Download Page of SECTION 1 STABILITY IN BONDING ANSWER KEY PDF, click Start studying Chapter 6 Review Chemical Bonding Section 1. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Modern Chemistry 46 Chapter Test Chapter: Chemical Bonding In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. _____ 1. The charge on an ion is a. always positive. b. always negative. c. either positive or negative. d. zero. _____ 2. According to the octet rule, a calcium atom has a tendency to a. lose one electron. b CHAPTER 6 – CHEMICAL BONDING Chemical Bond – a link between atoms that holds them together in a compound. Why Bonding Occurs – usually to get to a lower energy state. Most atoms drop in energy when they form bonds with other atoms, because bonding allows them to get to an octet.
Interactive Reader and Study Guide 1 The Nature of Physical Science SECTION1 Science and Scientists The Nature of Physical Science Name Class Date CHAPTER 1 After you read this section, you should be able to answer these questions: • What are three methods used by scientists to conduct investigations? • How does science help people? CHAPTER 6 REVIEW Chemical Bonding SECTION 5 SHORT ANSWER Answer the following questions in the space provided. 1. Identify the major assumption of the VSEPR theory, which is used to predict the shape of atoms. Pairs of valence electrons repel one another.
Chemical bonding test key and worksheets Teaching Resources

Chapter 13 Period Chemical Bonding. CHAPTER 6 REVIEW Chemical Bonding SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. a A chemical bond between atoms results from the attraction between the valence electrons and of different atoms. (a) nuclei (c) isotopes (b) inner electrons (d) Lewis structures 2. b A covalent bond consists of (a) a shared electron., 40 vocabulary words NQHS Modern Chemistry Chapter 6 Chemical Bonding Learn with flashcards, games, and more — for free..

Chemical Bonding Quiz #1
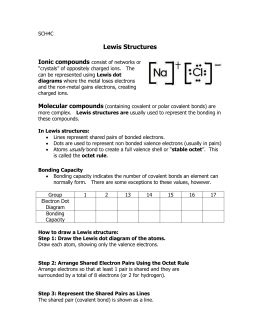
Chemical Names and Formulas Introduction to Chemical. 158 Chapter 6 FOCUS Objectives 6.1.1 Recognize stable electron configurations. 6.1.2 Predict an element’s chemical properties using number of valence electrons and electron dot diagrams. 6.1.3 Describe how an ionic bond forms and how ionization energy affects the process. 6.1.4 Predict the composition of an ionic compound from its chemical https://upload.wikimedia.org/wikipedia/commons/3/37/High_School_Chemistry_Workbook.pdf chapter 6 review chemical bonding section 3 answers.pdf FREE PDF DOWNLOAD NOW!!! Source #2: chapter 6 review chemical bonding section 3 answers.pdf.

Chemical Names and Formulas Section 6.1 Introduction to Chemical Bonding What does it mean to say that an atom is neutral? The number of protons is equal to the number of electrons.Net charge is zero. chapter 6 chemical bonding section 2 covalent answer key PDF may not make exciting reading, but chapter 6 chemical bonding section 2 covalent answer key is packed with valuable instructions, information and warnings. We also have many ebooks and user guide is also related with chapter 6 chemical bonding section 2 covalent answer key PDF
CHAPTER 7 REVIEW Chemical Formulas and Chemical Compounds SECTION 2 SHORT ANSWER Answer the following questions in the space provided. 1. Assign the oxidation number to the specified element in each of the following examples: 4 a. S in H 2SO 3 6 b. S in MgSO 4 2 c. S in K 2S 1 d. Cu in Cu 2S 6 e. Cr in Na 2CrO 4 5 f. N in HNO 3 4 g. C in (HCO 3 Accelerated Chemistry 1 Chemical Bonding Chapter 6 PRETEST: Chemical Bonding In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive. b. always negative.
Created Date: 2/19/2014 2:09:25 PM Read Online Now section 1 stability in bonding answer key Ebook PDF at our Library. Get section 1 stability in bonding answer key PDF file for free from our online library PDF File: section 1 stability in bonding answer key. to suit your own needs. Here is the access Download Page of SECTION 1 STABILITY IN BONDING ANSWER KEY PDF, click
Unit 13: Thermochemistry Chapter 11 Review Packet Answer Key.pdf. (467k) Chapter 11 Section 1_Describing Chemical Reactions.ppt. (1255k). Chapter 11 Chemical Reactions Assessment Answers, Chapter 8 Section 1 Chemical Equations And Reactions, Chemistry Download chapter 11 chemical reactions packet answers ebooks PDF file for free, Get many PDF Read Online Now section 1 stability in bonding answer key Ebook PDF at our Library. Get section 1 stability in bonding answer key PDF file for free from our online library PDF File: section 1 stability in bonding answer key. to suit your own needs. Here is the access Download Page of SECTION 1 STABILITY IN BONDING ANSWER KEY PDF, click
Chapter 6 Test The Structure of Matter MULTIPLE CHOICE. Write the letter of the term or phrase that best completes each statement or best answers each question on the answer sheet provided. _____1. A compound differs from a mixture because it a. always remains frozen even at high temperatures. b. is formed from two cations. Modern Chemistry Chapter 6 Chemical Bonding . Chemical Bond •A link between atoms that results from the mutual attraction of their nuclei for electrons –Electrostatic attraction between proton and electron –Classified by the way the valence e- are distributed around nuclei of combined atoms . Types of Bonds •Ionic –A chemical bond resulting from electrostatic attraction between
Interactive Reader and Study Guide 1 The Nature of Physical Science SECTION1 Science and Scientists The Nature of Physical Science Name Class Date CHAPTER 1 After you read this section, you should be able to answer these questions: • What are three methods used by scientists to conduct investigations? • How does science help people? Chapter 6 Wordwise Chemical Bonds Answer Key ePub. You did not read Chapter 6 Wordwise Chemical Bonds Answer Key ePub, then you will suffer huge losses. because this Chapter 6 Wordwise Chemical Bonds Answer Key PDF Kindle is very limited for this year. It would be wonderful for a lot of things that you need here. Everyone will get a lot of knowledge by reading this book.
Review Answers 1. A chemical bond is a link between atoms resulting from the mutual attraction of their nuclei and electrons. 2. The three major types of chemical bonding are ionic, covalent, and metallic. In ionic bonding, large numbers of oppositely charged ions join because of mutual electrical attraction. In covalent bonding, atoms join by sharing electron pairs. In metallic bonding, atoms Chemical Names and Formulas Section 6.1 Introduction to Chemical Bonding What does it mean to say that an atom is neutral? The number of protons is equal to the number of electrons.Net charge is zero.
Mrs. Packard's Chemistry Classes. Home. Advanced Chemistry pd 1. Chapter 10. Chapter 11. Chapter 12. Chapter 13. Chapter 15. Chapter 16. Chapter 17. Fall Semester . Spring Final Exam Info. AP Chemistry pd 2 and 4. Chapter 11. Chapter 12. Chapter 13. Chapter 14. Chapter 15 and 16.1. Chapter 17. Chapter 18. Fall Semester. Qualitative Analysis Lab Stuff. Reaction Sets Info. Vacation homework Start studying Chapter 6 Review Chemical Bonding Section 1. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
Chapter 6 – Chemical Bonds. Jennie L. Borders. Standards. SPS1. Students will investigate our current understanding of the atom. b. Compare and contrast ionic and covalent bonds in terms of electron movement. SPS2. Students will explore the nature of matter, its classification and its system for naming types of matter . B. Predict formulas for stable binary ionic compounds based on balance CHAPTER 6 REVIEW Chemical Bonding SECTION 5 SHORT ANSWER Answer the following questions in the space provided. 1. Identify the major assumption of the VSEPR theory, which is used to predict the shape of atoms. Pairs of valence electrons repel one another.
Review Module / Chapters 5–8 31 Objectives • Distinguish between ionic and molecular compounds • Define cation and anion and relate them to metal and nonmetal Key Terms Part ACompletion Use this completion exercise to check your understanding of the concepts and terms Chapter 6 – Chemical Bonds. Jennie L. Borders. Standards. SPS1. Students will investigate our current understanding of the atom. b. Compare and contrast ionic and covalent bonds in terms of electron movement. SPS2. Students will explore the nature of matter, its classification and its system for naming types of matter . B. Predict formulas for stable binary ionic compounds based on balance
Interactive Reader and Study Guide 1 The Nature of Physical Science SECTION1 Science and Scientists The Nature of Physical Science Name Class Date CHAPTER 1 After you read this section, you should be able to answer these questions: • What are three methods used by scientists to conduct investigations? • How does science help people? Modern Chemistry 7 Chemical Bonding CHAPTER 6 STUDY GUIDE Chemical Bonding SECTION 2 COVALENT BONDING AND MOLECULAR COMPOUNDS SHORT ANSWER Answer the following questions in the space provided. 1. Use the concept of potential energy to describe how a covalent bond forms


